FRAMINGHAM, MA — Batches of Irbesartan Tablets and Irbesartan and Hydrochlorothiazide Tablets made by Lupin Pharmaceuticals Inc. are being recalled due to concern with cancer causing impurities in the medication.
Testing of the drug during manufacturing revealed an impurity which called “N-nitrosoirbesartan” which is a probable human carcinogen (a substance that could cause cancer).
Lupin is recalling all batches of Irbesartan Tablets USP 75mg, 150mg and 300mg and Irbesartan and Hydrochlorothiazide Tablets USP, 150mg/12.5mg and 300mg/12.5mg in the US.
From October 8, 2018 (the earliest date of shipment from the manufacturing site of any of the affected batches), to September 30, 2021, Lupin received 4 reports of illness from Irbesartan and 0 reports from Irbesartan and Hydrochlorothiazide.
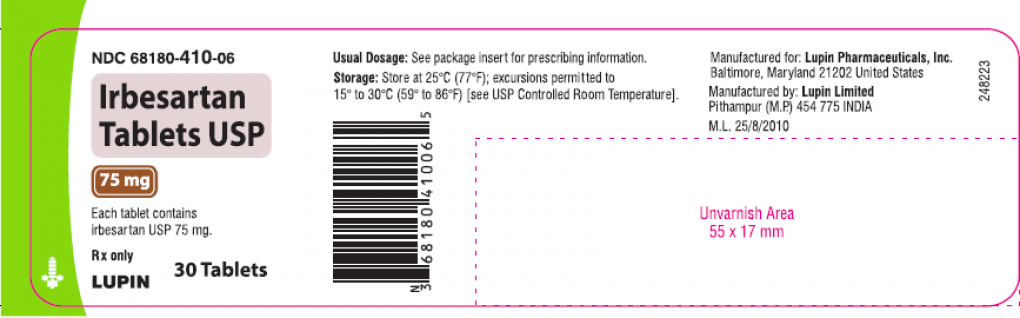
Irbesartan tablet USP is an angiotensin II receptor blocker indicated for treatment of hypertension, to lower blood pressure, diabetic nephropathy in hypertensive patients with type 2 diabetes, an elevated serum creatinine, and proteinuria.
Irbesartan Tablets USP 75mg, 150mg and 300mg is packaged in 30 and 90 count bottles and was distributed nationwide in the US to wholesalers, drug chains, mail order pharmacies and supermarkets.

The recall was announced on the U.S. Food & Drug Administration (FDA) website on October 14, 2021. The announcement includes detailed information about lot numbers, dates and product specific labels.
The announcement also includes information about how to contact the FDA to report adverse reactions or quality problems experienced with the use of this product to the FDA’s MedWatch Adverse Event Reporting program online, by regular mail or by fax.
You can find the FDA Announcement here.
Information about how to return unused prescriptions of these drugs and how to obtain refunds are also included in the FDA notice.
###
